You may have experienced urine leakage for years. But did you know that there’s a lasting, effective solution for the source of your symptoms?
There’s a way to get you back out there – running, jumping, laughing, coughing, sneezing – and worrying less about leaks.
How Altis works
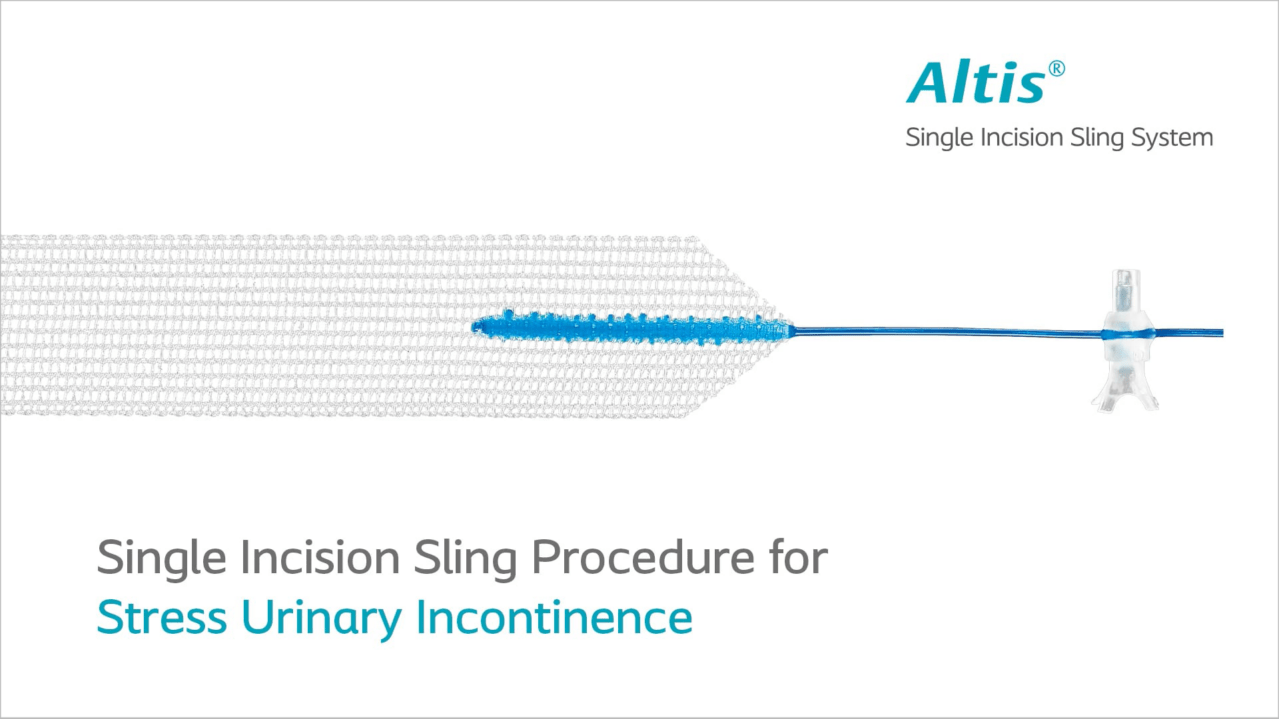
Altis is an implanted device used during a minimally invasive procedure that treats stress urinary incontinence at its source. In many cases, the surgery takes 30 minutes or less1 and is typically an outpatient procedure.
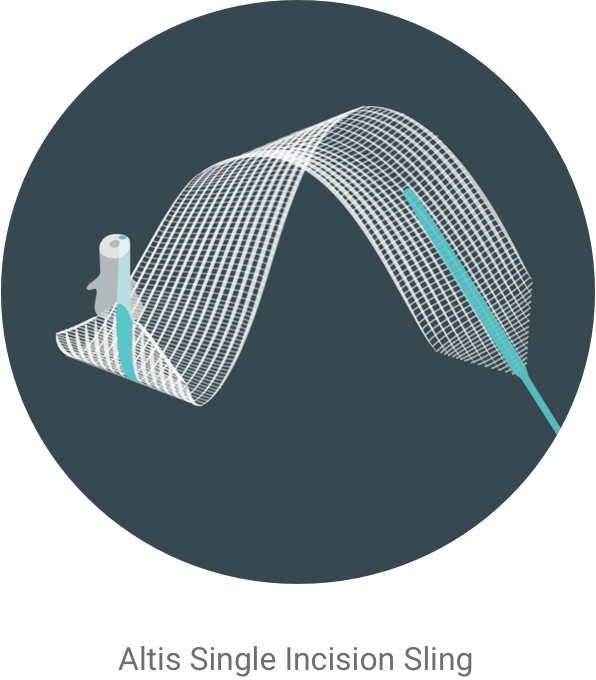
During the procedure, your doctor will make one small (typically 1-2 cm) incision to place an Altis sling beneath your urethra; this provides support to the urethra when there is stress on the bladder, such as when you cough, laugh or sneeze.

After the procedure, most women experience less leaks.2 The procedure to implant the Altis Single Incision Sling results in two less incisions, and less post-op pain compared to traditional slings.3 This means a quicker recovery – on average 5 days sooner with single incision slings compared to traditional slings.3
What to expect before, during and after surgery
Before surgery – Medical history
First, your doctor will collect and review your medical history and recent experiences. You will be asked to provide a list of all medications and supplements you take, and information about your urinary habits and normal fluid consumption. It’s important to accurately describe the leakage you are having, such as when and under what conditions leakage occurs.
Before surgery – Physical exam
Then, you’ll likely have a physical exam, which can include:
- A cough stress test where you will be asked to cough and bear down with a full bladder to see if urine leaks
- An “at home pad test” to help estimate how much you are leaking throughout the day to determine the severity of your urinary incontinence4
- Urinalysis – testing of a urine sample
- Post-void residual – measures the amount of urine left in your bladder after urinating
- Cystoscopy – use of a scope to examine your bladder
- Urodynamics – testing that measures amount of urine in the bladder before urinating and the force of the urine as it leaves the body
Based on the results of your physical exam, you and your doctor will discuss and decide the right solution for your specific needs.
During surgery
A sling procedure is the most common minimally invasive surgical option to correct stress urinary incontinence. It is usually performed as an outpatient procedure in less than 30 minutes.
Slings are placed through a small incision in the vagina and placed under the urethra. Once placed, the sling will provide support for the urethra and help prevent leaks during physical activity such as coughing, laughing, or exercise.
After surgery and into recovery
Your doctor will provide you with information about your recovery. Generally, following your sling placement, expect to avoid physical strain, sexual intercourse, and heavy lifting for 6 weeks. You’ll likely be able to resume other normal activities after 2 weeks.
Contact your physician if you experience any bleeding, pain, or any signs of infection.



Women receiving single incision slings reported lower post-op pain, earlier return to normal activities, and earlier return to work compared to those receiving traditional slings.3
FAQs
Urinary incontinence 101
What is urinary incontinence?
Urinary incontinence happens when a person loses voluntary control over their urinary functions.
Approximately 78 million women in the U.S. suffer from urinary incontinence.5
78 million
women in the U.S. suffer from urinary incontinence.5
Are there different types of urine leakage?
The most common types are stress urinary incontinence, urge urinary incontinence and mixed urinary incontinence.4
- Stress urinary incontinence (SUI) happens when urine leaks during coughing, laughing or exercise because the urethra does not function properly.4
- Urge urinary incontinence (UUI), also known as overactive bladder (OAB), involves the sudden sensation of the need to urinate that can be hard to put off — the sudden urge to go.4
- Mixed urinary incontinence (MUI) is a combination of stress and urge incontinence.4
What causes stress urinary incontinence?
SUI generally occurs when your pelvic muscles are not strong enough to keep the opening of the bladder neck closed when there’s pressure on your bladder. It can slowly develop as you age and may be the result of a specific event such as childbirth, or be a result of chronic smoking, constipation, obesity, aging, heavy lifting, or genetics.6
SUI treatment options
What are my treatment options?
Treatment options for stress urinary incontinence range from the day-to-day management of symptoms to surgical treatments that provide a more permanent solution. Examples of non-surgical options include wearing pads or absorbent underwear, lifestyle changes, vaginal pessaries, or improving pelvic strength through muscle exercises.6
Surgical treatments for SUI include a sling procedure and injectable bulking agents.6
Can stress urinary incontinence be successfully treated?
Yes, SUI can be treated successfully.2
Talk to your doctor to discuss what treatment might be right for you.
What kind of doctor should I talk to?
Less than 50% of women with SUI discuss their symptoms with their doctor.7 That’s why finding the right physician to treat your SUI is a very important step in seeking treatment. It’s important that your doctor listens to you, advocates for your best interest, and provides the right path for your specific needs. Specialists that typically treat SUI are urogynecologists, urologists, and gynecologists.
What is stress urinary incontinence surgery?
Stress urinary incontinence surgery may be approached in different ways.1 Altis is a minimally invasive option that uses a synthetic material to help support your urethra, which is commonly known as a sling. It helps cradle the urethra by providing additional support to help treat urinary incontinence.1 In some cases, the surgeon may use your own tissue to treat your incontinence.
Are there different types of slings?
There are many different types of slings available and a variety of ways to place a sling.1 A sling is chosen for treatment based on several factors, including the severity of incontinence and prior pelvic surgery, as well as patient and doctor preferences.
Coloplast offers a variety of slings. The synthetic slings are made from polypropylene mesh that, when implanted, combines with new tissue growth to become a support structure for the urethra.
Regardless of type, all slings are placed through the vagina through a small incision under the urethra. There may be other small incisions within the groin creases or by the pubic hairline, depending on the type of sling placed. Like a backboard, the sling supports the urethra during activities that put a strain on the area and prevents leakage from occurring.1
SUI surgical treatment outcomes and recovery
Will I be able to feel the sling?
It’s possible. Every person’s physician anatomy is different, so everyone experiences the sling differently.
Are there any risks?
Like any surgery, there are some risks related to the procedure. Talk to your doctor to discuss these risks in detail.
See important safety information and the following links for more information.
What is the recovery time?
Maybe you have been putting off a surgical option for treating your leaks because you can’t afford to be out of commission. Work needs you. Your family needs you.
Your doctor will provide you with information about your recovery. Generally, following your sling placement, expect to avoid physical strain, sexual intercourse, and heavy lifting for 6 weeks, but you’ll likely be able to resume other normal activities after 2 weeks.
SUI surgical treatment cost and coverage
What does this cost? Will my insurance cover stress incontinence surgery?
Most insurance plans, including Medicare, cover these procedures.
Consult your insurance carrier to find out the specific criteria for coverage. The reimbursement specialist at your physician’s office may also be able to help you with this.
Not sure where to start?
Take our short, free, and confidential self-assessment.
Help is on the way.