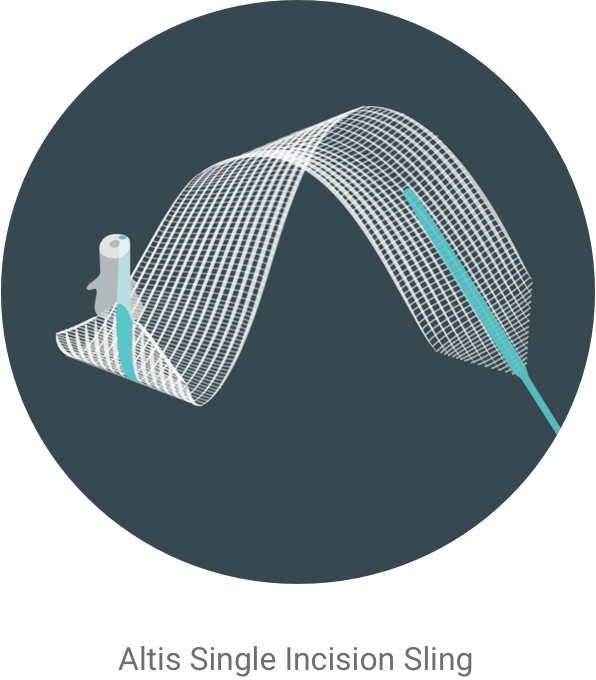
Fugas de vejiga
La pérdida de la vejiga o la pérdida de orina es común. Si tiene problemas con la pérdida de la vejiga, no está sola.
Encuentre la solución a la incontinencia
El camino para llegar a un diagnóstico o a una solución no es el mismo para todas las personas. La experiencia de cada mujer con la incontinencia urinaria de esfuerzo (IUE) es diferente y puede llegar a estos pasos a ritmos distintos y durante etapas diferentes de su vida.
¿Cuáles son las causas del desarrollo de la incontinencia urinaria de esfuerzo (IUE)?
¿Cuáles son las causas de la IUE?
Más información

Existen diferentes formas de abordar el control de los síntomas.

El médico adecuado la escuchará y pondrá sus necesidades en primer lugar. Pero ¿cómo puede encontrarlo?

¿Sufre de incontinencia urinaria de esfuerzo?
Los músculos de la uretra funcionan como una válvula: se abren y se cierran según sea necesario para dejar salir la orina. Pero en la incontinencia urinaria de esfuerzo, también llamada IUE, los músculos pélvicos que normalmente sostienen la vejiga y la uretra están debilitados.
Cuando esto ocurre, la orina sale de la vejiga y esto puede hacerla sentir avergonzada, frustrada e insegura acerca de lo que le está sucediendo.
Síntomas de la incontinencia urinaria
de esfuerzo
¿Tiene pérdidas durante alguna de las siguientes actividades?
- Reír
- Toser
- Estornudar
- Levantamiento de objetos pesados
- Actividad física
- Relaciones sexuales
Si responde “sí” a una o más opciones, debería consultar a un médico que esté familiarizado con la IUE y hablar sobre una solución más permanente para tratar las pérdidas de orina.

¿Cuáles son las causas de la IUE?
La IUE se puede desarrollar lentamente a medida que envejece y también puede ser el resultado de un acontecimiento específico, como el parto, o ser consecuencia de fumar, obesidad u otros traumatismos previos de los tejidos en el área.2
¿Cuáles son sus opciones
de tratamiento?
Seguramente ya probó de todo. Desde toallitas, pañales, ropa interior especial y ejercicios de Kegel. Algunas de estas opciones ayudan, pero no alivian la carga de la IUE. Se merece un tratamiento que aborde el origen del problema, no algo que se limite a brindar una solución temporal para los síntomas.

Evitar y encubrir las pérdidas no hace sino aumentar la carga que supone tener IUE en primer lugar. Una solución duradera, con un tiempo de inactividad mínimo, puede ayudar a aliviar la carga añadida de ocultar constantemente sus síntomas de IUE.
Por suerte, existe el cabestrillo de incisión única Altis®.
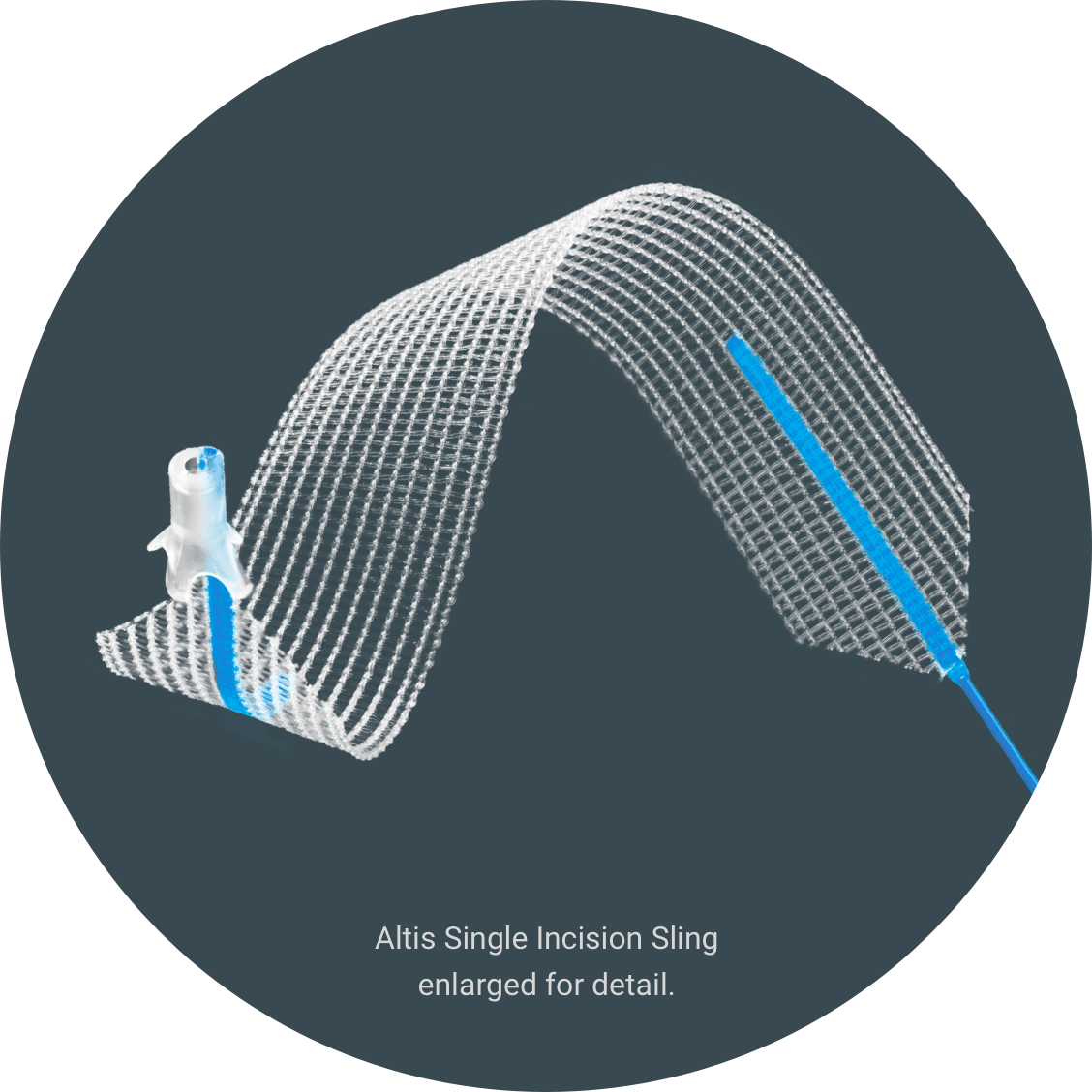
Presentación de Altis®
Las mujeres con IUE merecen una solución eficaz y clínicamente probada. Altis es un tratamiento que cuenta con el respaldo de estudios clínicos con testimonios de primera mano de mujeres que se han sometido al procedimiento con resultados satisfactorios.
Altis es un cabestrillo que trata la incontinencia urinaria de esfuerzo sosteniendo la uretra para mantenerla en su posición correcta.3 El procedimiento es una cirugía mínimamente invasiva4 ambulatoria.3
¿Qué han experimentado otras personas?
0 %
experimentó una reducción media del peso de la toallita4
0 %
declaró que se sentía “muchísimo mejor” o “mucho mejor” tras la cirugía a los
24 meses4
0 %
informó que no tuvo pérdidas de orina relacionadas con la IUE a los 24 meses después del procedimiento4
Encontrar a un especialista
Busque un especialista en cirugía pélvica cerca de donde vive.

Recupere la normalidad
Es fundamental encontrar un médico con la experiencia y la empatía necesarias para tratar su afección. Probablemente, se pregunte: “¿Hay algún especialista en cirugía pélvica cerca de donde vivo?”. Nosotros nos encargamos.
Sólo tiene que introducir su código postal para ver un listado de los médicos especialistas en pelvis de su área. En cada lista, figura el nombre del médico y su especialización en el área de salud pélvica, junto con su sitio web, número de teléfono y distancia desde su hogar.
Coloplast ha creado y mantiene este directorio. Su objetivo es ayudar a los pacientes que deseen obtener más información sobre las opciones de tratamiento de la incontinencia urinaria de esfuerzo (IUE) y el prolapso de órganos pélvicos (POP), poniéndolos en contacto con médicos que hayan demostrado calificaciones e interés en brindar una atención de calidad para estas afecciones. Los nombres y datos de este directorio se brindan únicamente a título informativo. Las decisiones relativas a la elección del médico y el tratamiento son responsabilidad del paciente, al igual que toda comunicación e interacción con los profesionales médicos de la lista. La información que envíe a un médico no está cubierta por la política de privacidad de Coloplast.
Los médicos que figuran en el localizador de médicos no pagan para que se los incluya. Algunos médicos de esta lista pueden comprar productos a Coloplast, prestarle servicios de consultoría o ser parte de un acuerdo de marketing conjunto con Coloplast. Coloplast no ofrece ninguna declaración ni garantía en relación con las competencias o el nivel de habilidad de ninguno de los médicos que figuran en el localizador de médicos ni con la calidad de los resultados de sus procedimientos, ni será responsable de ello. Usted y su médico deben determinar el procedimiento adecuado para usted.
Para obtener más información sobre este buscador de médicos o si tiene alguna pregunta sobre como añadir o gestionar los listados del directorio, póngase en contacto con el administrador.
Información importante sobre seguridad
Información importante sobre seguridad
La incontinencia urinaria de esfuerzo es una afección en la que la orina se escapa involuntariamente al ejercer presión en la vejiga (por ejemplo, al toser, estornudar, levantar objetos pesados o hacer ejercicio). La incontinencia urinaria de esfuerzo puede tratarse con un procedimiento quirúrgico en el que se implanta un cabestrillo de malla que funciona como una “hamaca” para sujetar la uretra, el conducto que se conecta con la vejiga y que lleva la orina al exterior del cuerpo. Una cirugía para colocar un cabestrillo para la incontinencia se realiza bajo anestesia y puede necesitar pasar la noche en el hospital.
El sistema de cabestrillo de incisión única Altis está indicado para el tratamiento de la incontinencia urinaria de esfuerzo (IUE) femenina provocada por el cierre incorrecto de la uretra (hipermovilidad uretral) o la debilidad del esfínter uretral (deficiencia intrínseca del esfínter [DSI]).
El médico debe informarle que el sistema de cabestrillo de incisión única Altis no está indicado para mujeres en los siguientes casos: * Que estén embarazadas o deseen quedar embarazadas. * Que tengan potencial de crecimiento futuro (p. ej., adolescentes). * Que tengan una infección de las vías urinarias activa conocida o una infección en el campo operatorio. * Que toman medicación anticoagulante (terapia anticoagulante). * Que tienen una uretra anormal (p. ej., fístula, divertículo). * Que tienen una afección, inclusive una patología pélvica conocida o presunta, que pudiera poner en peligro el implante o su colocación. * Que tienen sensibilidad o alergia al polipropileno o poliuretano.
Consulte con el médico lo siguiente:
- Las razones para elegir un cabestrillo de malla, incluidas las advertencias, las precauciones y los riesgos asociados con su uso.
- Los tratamientos alternativos para la incontinencia que pueden ser adecuados.
- El cabestrillo Altis que se implanta es permanente.
- Complicaciones graves asociadas con la malla pueden requerir una o varias cirugías de revisión.
- La extracción parcial o completa de la malla puede que no siempre sea posible o aconsejable, ya que puede que no corrija estas complicaciones por completo.
- Pueden ocurrir complicaciones nuevas (de novo) y la IUE puede reaparecer o empeorar.
- Puede haber dolor persistente, con extracción de la malla o sin extracción, y puede presentarse distintos grados de cicatrización.
- Ciertas afecciones subyacentes pueden ser más propensas a hemorragias posquirúrgicas, alteraciones del suministro de sangre, problemas o retrasos en la cicatrización, exposición del cabestrillo de malla u otras complicaciones.
Se deben tener en cuenta los posibles riesgos y beneficios adicionales de Altis en pacientes con una o más de las siguientes afecciones: * afecciones subyacentes relacionadas con la edad * enfermedad autoinmune * trastorno de la coagulación * trastorno del tejido conectivo * estado debilitado o inmunocomprometido * diabetes * radioterapia pélvica o quimioterapia * características físicas (p. ej., índice de masa corporal) * insuficiencia renal * afecciones subyacentes relacionadas con el tabaquismo * anomalías de las vías urinarias
Cualquier embarazo futuro podría anular los beneficios de este procedimiento quirúrgico.
Las pacientes deben informar cualquier sangrado, dolor, flujo vaginal anormal o signos de infección que ocurran en cualquier momento.
Se sabe que pueden ocurrir complicaciones, las cuales pueden ser inmediatas o tardías, localizadas o sistémicas, de aparición nueva (de novo) o un empeoramiento, agudas o crónicas, transitorias o permanentes, de aparición nueva (de novo) o continuas, en empeoramiento, transitorias o permanentes.
Las posibles complicaciones pueden incluir, entre otras, las siguientes:
|
|
Este tratamiento está indicado por el médico. Analice las opciones de tratamiento con el médico para comprender los riesgos y beneficios de las distintas opciones a fin de determinar si el cabestrillo de malla es la opción adecuada para usted.
Precaución: La ley federal (EE. UU.) exige que la venta de este dispositivo esté a cargo de un médico o se realice en función del pedido de un médico.
Minneapolis, MN
PM-37734 04.2025
Referencias:
- Patel, U. J., Godecker, A. L., Giles, D. L., & Brown, H. W. (2022). Updated Prevalence of Urinary Incontinence in Women: 2015-2018 National Population-Based Survey Data. Female pelvic medicine & reconstructive surgery, 28(4), 181–187. https://doi.org/10.1097/SPV.0000000000001127
- Abrams, P., Andersson, K. E., Apostolidis, A., Birder, L., Bliss, D., Brubaker, L., Cardozo, L., Castro-Diaz, D., O’Connell, P. R., Cottenden, A., Cotterill, N., de Ridder, D., Dmochowski, R., Dumoulin, C., Fader, M., Fry, C., Goldman, H., Hanno, P., Homma, Y., Khullar, V., … members of the committees (2018). 6th International Consultation on Incontinence. Recommendations of the International Scientific Committee: EVALUATION AND TREATMENT OF URINARY INCONTINENCE, PELVIC ORGAN PROLAPSE AND FAECAL INCONTINENCE. Neurourology and urodynamics, 37(7), 2271–2272. https://doi.org/10.1002/nau.23551
- Surgery for stress urinary incontinence. ACOG. (n.d.). Retrieved December 2, 2022, from https://www.acog.org/Patients/FAQs/Surgery-for-Stress-Urinary-Incontinence
- Kocjancic, E., Erickson, T., Tu, L. M., Gheiler, E., & Van Drie, D. (2017). Two-year outcomes for the Altis® adjustable single incision sling system for treatment of stress urinary incontinence. Neurourology and urodynamics, 36(6), 1582–1587. https://doi.org/10.1002/nau.23156