Pelvic organ prolapse
Instead of living with POP, live better without it.
What is prolapse?
Up to 50% of women will experience pelvic organ prolapse.1
Pelvic organ prolapse (POP) is the dropping of the pelvic organs caused by the loss of normal support of the vagina. It occurs when there is weakness or damage to the normal support of the pelvic floor causing pelvic organs (the vagina, cervix, uterus, bladder, urethra, intestines or rectum) to drop down. Women with POP may feel or see a bulge coming out of the opening of their vagina. 2
Experiencing pelvic organ prolapse can be challenging — it can interfere with your activities, intrude on your personal life, and cause some serious discomfort.2 However, there are effective solutions3 that can repair POP at the source, so you can get back to living the life you want.
Find your path to prolapse relief
No two people walk the same path to a diagnosis or solution. Every woman’s prolapse journey is different, and they may reach these steps at different paces and during different times in their lives.
Are the symptoms you’re experiencing more than just an inconvenience? Learn about the signs and causes of pelvic organ prolapse.

The right doctor will listen to you and put your needs first. But how do you find them?
Finding the right provider

It is important to understand your body and learn about all of your treatment options, both non-surgical and surgical, to help find a solution that is right for you.
Explore options

Find out more about post-op downtime and what to expect after heading home.
Healing and heading home

Types of prolapse
There are several different types of pelvic organ prolapse, with different names depending on the organs involved.4
Female pelvic anatomy without prolapse
Anterior Vaginal Wall Prolapse (Cystocele)
occurs when there is a loss of support to the front wall of the vagina. The bladder drops down and may cause a bulge in the vaginal opening.Posterior Wall Prolapse (Rectocele or Enterocele)
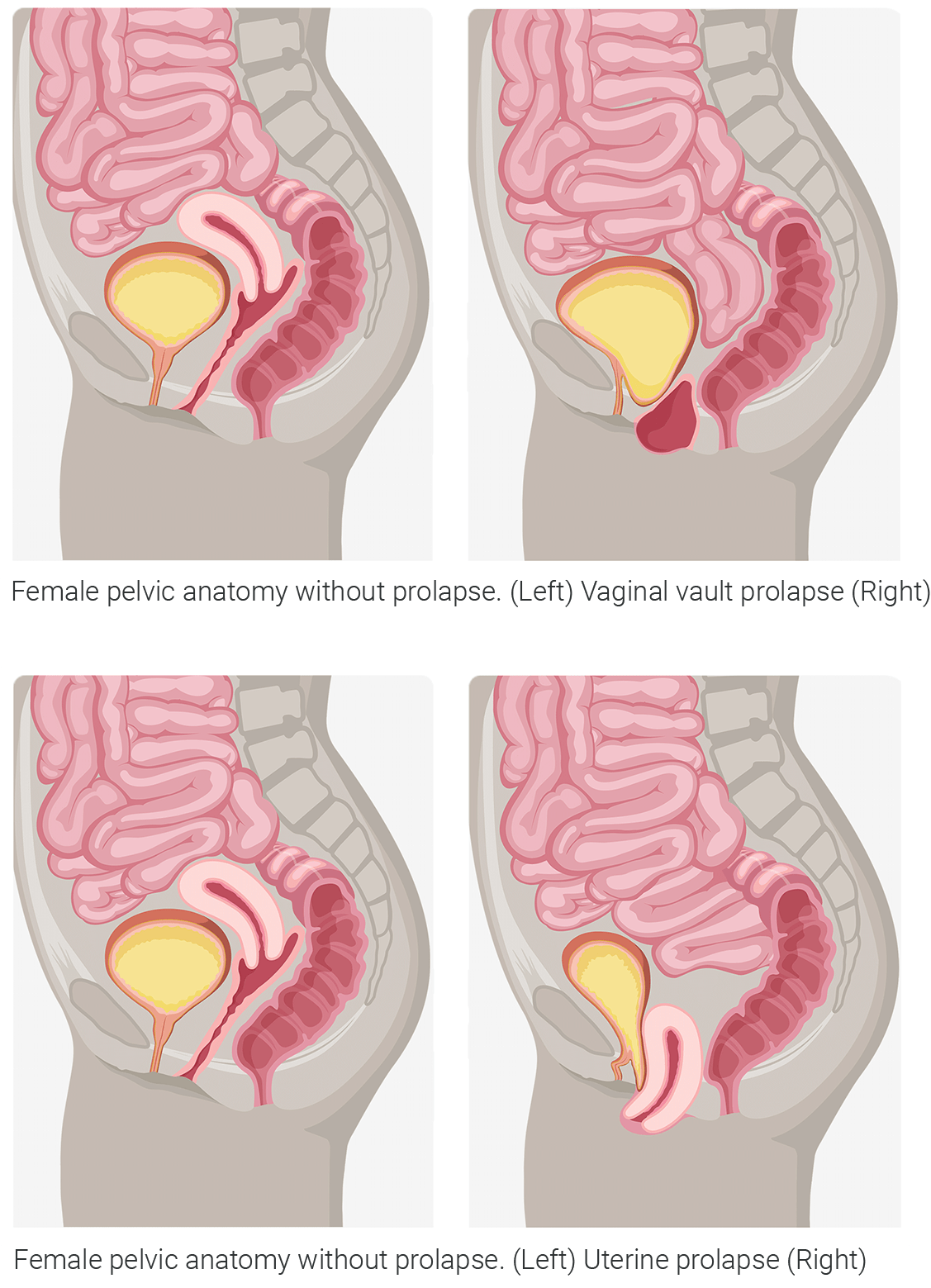
occurs when there is a loss of support to the back wall of the vagina. The rectum or intestines drops down and may cause a bulge in the vaginal opening.Apical Prolapse (Vaginal vault or Uterine)
occurs when there is a loss of support to the uterus and the top part of the vagina after a hysterectomy. These types of prolapse are often associated with the loss of anterior or posterior vaginal wall support.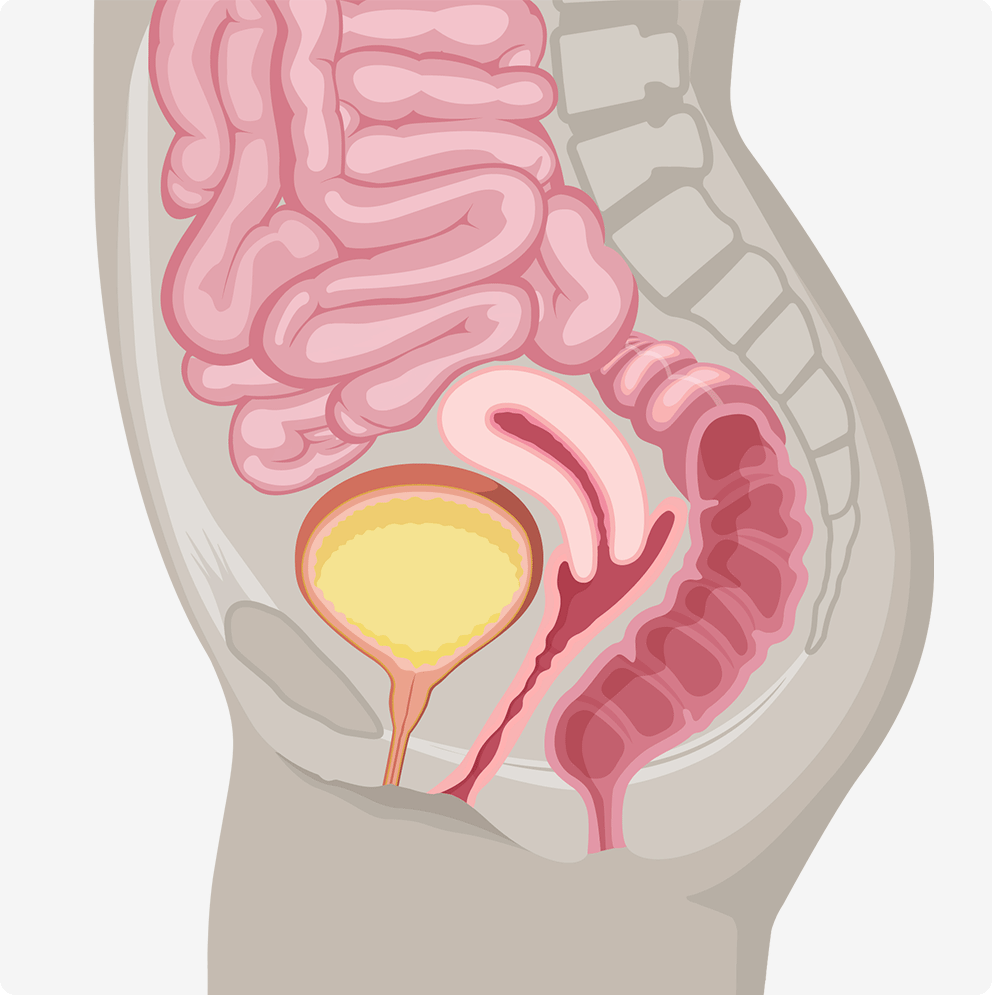
Female pelvic anatomy without prolapse
 Female pelvic anatomy without prolapse
Female pelvic anatomy without prolapse 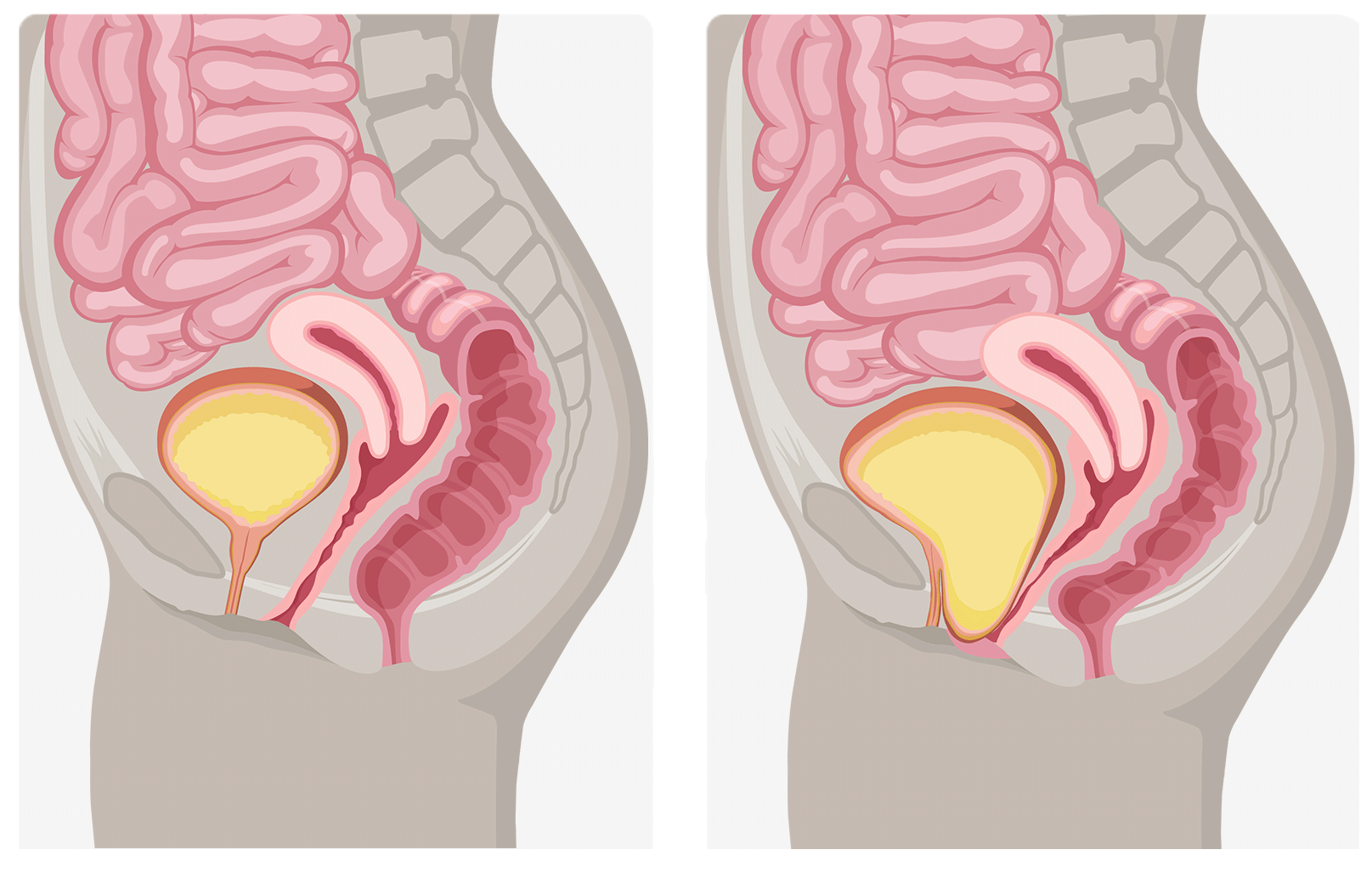 Female pelvic anatomy without prolapse. (Left) Cystocele prolapse (Right)
Female pelvic anatomy without prolapse. (Left) Cystocele prolapse (Right) 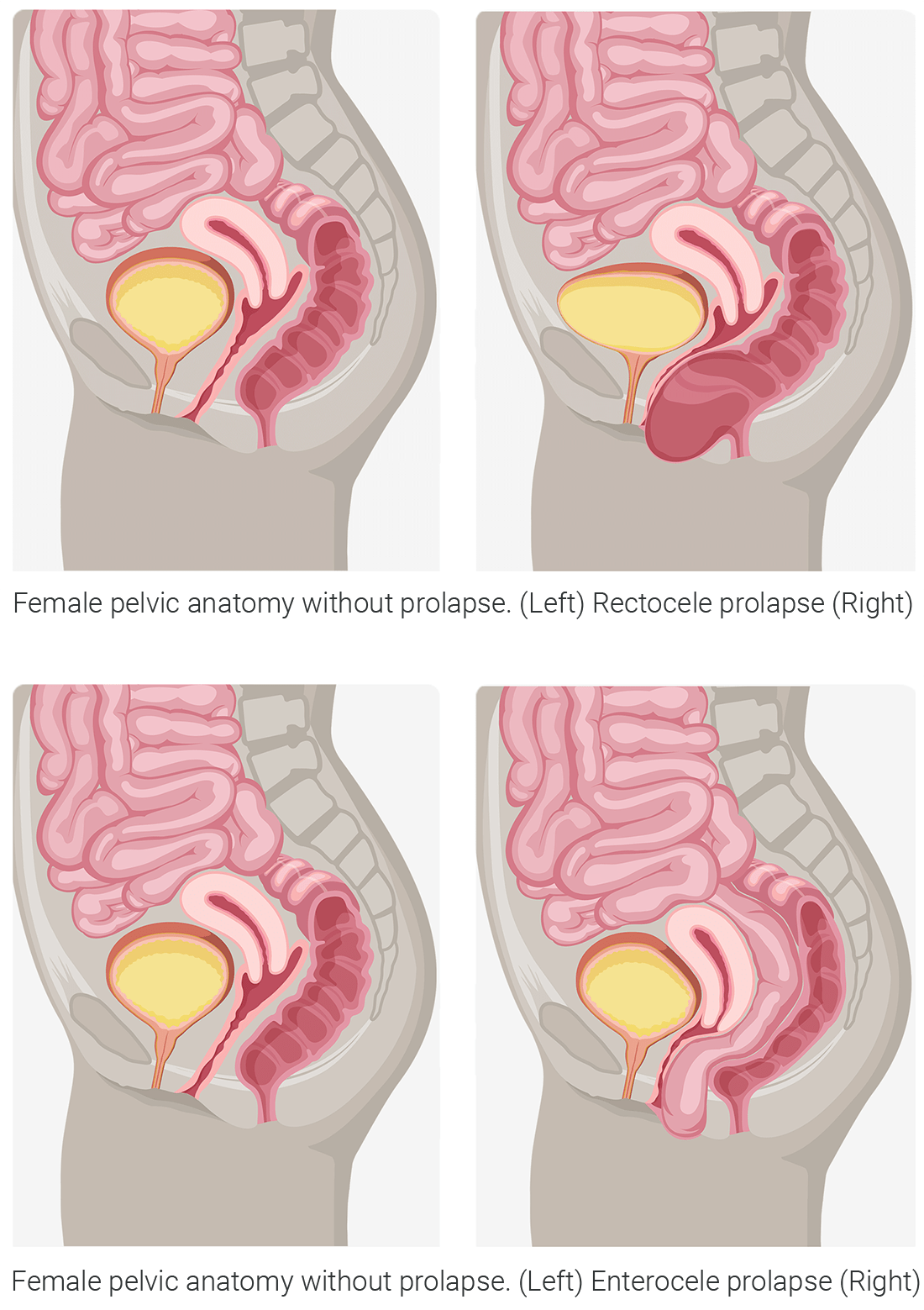
Symptoms of prolapse
While it is not life-threatening, women with prolapse experience symptoms that impact their day-to-day lives and keep them from experiencing moments to the fullest. But these symptoms won’t always seem obvious. As prolapse progresses, the symptoms may become more apparent and painful. If you are experiencing prolapse, you may feel:
- Pressure in the pelvic region, vaginal discomfort, pain or bleeding4
- Pulling or aching in the lower abdomen or pelvis, a bulge coming out of the vagina4
- Pain or discomfort during sex5
- Difficulty urinating or having a bowel movement4
Symptoms of prolapse
While it is not life-threatening, women with prolapse experience symptoms that impact their day-to-day lives and keep them from experiencing moments to the fullest. But these symptoms won’t always seem obvious. As prolapse progresses, the symptoms may become more apparent and painful. If you are experiencing prolapse, you may feel:
- Pressure in the pelvic region, vaginal discomfort, pain or bleeding4
- Pulling or aching in the lower abdomen or pelvis, a bulge coming out of the vagina4
- Pain or discomfort during sex5
- Difficulty urinating or having a bowel movement4
What causes prolapse?
Pelvic organ prolapse can drastically impact your lifestyle. Things that can cause the muscles in the pelvis to become stretched or weakened include:
- Pregnancy and childbirth2
- Genetics2
- Smoking2
- Pelvic Floor Injury2
- Chronic constipation2
- Chronic coughing2
- Obesity2
- Menopause2
- Nerve and muscle diseases2
What causes prolapse?
Pelvic organ prolapse can drastically impact your lifestyle. Things that can cause the muscles in the pelvis to become stretched or weakened include:
- Pregnancy and childbirth2
- Genetics2
- Smoking2
- Pelvic Floor Injury2
- Chronic constipation2
- Chronic coughing2
- Obesity2
- Menopause2
- Nerve and muscle diseases2

Understanding treatment options
There’s nothing simple about dealing with prolapse, but there are treatment options. Your physician may recommend some of these non-surgical options as a first step to relief:
- Vaginal pessary: a removeable device placed in the vagina to support the pelvic floor and support the prolapsed organ. Your physician will fit and insert the pessary, which must be cleaned frequently and may have to be removed before intercourse.6
- Kegels: an exercise you can do on your own or with the guidance of a pelvic floor therapist to help strengthen your pelvic floor muscles.7
- Biofeedback therapy: a technique that uses different types of devices to give information on how well pelvic muscles are contracting. This information can help improve awareness and control of pelvic floor muscles.7
Non-surgical options may involve long-term treatment, ongoing maintenance and continued expenses, and may not address the underlying condition.
Non-surgical options aren’t the only approach.
You have the option of surgical solutions backed by over 15 years of experience and clinical data with firsthand testimonials from those who’ve undergone POP procedures and experienced successful outcomes.
The type of surgery you get depends on the type of prolapse you have. Not every solution is right for every person – treatment decisions must be unique to each individual’s medical history, symptoms and lifestyle.
Finding the right doctor who will recommend the right procedure based on your needs will help make sure you’re on the right path.

What are the outcomes?
Women experiencing POP deserve a clinically proven and effective solution that lasts.
Prolapse repair procedures have effective outcomes.
0 %
of patients were “satisfied”
or “very satisfied”8
0 %
of patients stated they
would definitely “do it all over again” if they had the chance8
0 %
stated they “would definitely recommend to a friend”8
Patient spotlight
Laurie’s story
Laurie first heard the words “cystocele,” “rectocele” and “pelvic organ prolapse” before her symptoms started, during a regular annual exam with her gynecologist.





